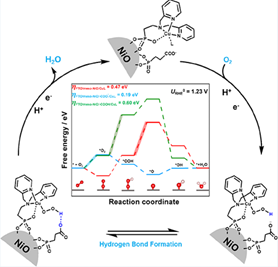
Figure 1. This scheme Illustrates efficient, selective oxygen reduction using earth-abundant electrocatalyst and new surface cocatalysts.
Ceaseless efforts from extensive research are ongoing to establish a sustainable and reliable future energy source. One of the promising technologies for promoting the switch from non-renewable to renewable sources is fuel cells. However, the sluggish oxygen reduction reaction (ORR) at the cathode hampers the overall electrochemical performance of a fuel cell by generating an unfavourable byproduct, which is H2O2. A research group led by Dr. Edmund CM TSE from the Department of Chemistry at the University of Hong Kong, has achieved an important breakthrough in dismantling the technological barrier of fuel cells. Specifically, the group has developed a promising electrochemical cocatalyst that overcomes the kinetic hindrances of ORR.
Furthermore, the research team explored the relationship between proton-coupled electron transfer steps and solution pH. 'At pH 7, the terminus of PPA converts from a neutral carboxylic acid group into an anionic carboxylate group.' Xutao Gao, a Ph.D. student in the Tse group, performed density functional theory (DFT) calculations (Figure 1) to understand the working principle of this newly discovered cocatalyst. 'The negatively charged group then enticed the intermediates to form interfacial hydrogen bonds, thereby lowering the ORR energy barrier and releasing H2O as the sole product.' Distinct from the comparative control systems, the carboxylate-terminated FTO/ meso-NiO/ PPA/ CuL catalyst showed a particularly low energy barrier of 0.19 eV due to the formation of hydrogen bonds. The study was published on ACS Catalysis in an article entitled 'Immobilization of a Molecular Copper Complex and a Carboxylate Terminated Cocatalyst on a Metal Oxide Electrode for Enhanced Electrocatalytic Oxygen Reduction.'
Link of journal paper can be accessed from: https://pubs.acs.org/doi/10.1021/acscatal.3c00384
