I. Method developing for Bio-macromolecules synthesis
Chemical tools have allowed for many biological question to be answered at a molecular level. One
long-term goal of this research program is to design and discover chemical methods enabling chemical
synthesis of bio-macromolecules, including proteins, carbohydrates, and proteins with
post-translational modifications. We are also engaged to development of fluorogenic reagents for
labeling proteins and carbohydrates and sensors for detecting bio-molecules under physiological
conditions.
II. Medicinal Chemistry of Natural Products
We are interested in the total synthesis of bioactive natural products and studies on their medicinal chemistry. We have completed the total synthesis of several antibiotics and we are engaging their medicinal chemistry now towards the development of next-generation antibiotics.
III. Function Definition of Macromolecules and their PTMs
Homogeneous proteins with site-specific glycosylation, phosphorylation, methylation, acetylation and other PTMs, whose generation is difficult via protein expression, can be readily obtained by protein chemical synthesis. So, we take advantage of organic synthesis and have established well-equipped biological laboratory for functional research of macromolecules.
IV. Drug Development
Super diverse peptide library provides a powerful tool for discovering bioactive peptides. We are using (mirror) phage peptide display and one-bead-one-compound to build super diverse libraries for drug discovery.
I. NEW Methods developed in our lab
1) Chemoselective peptide ligation for protein chemical synthesis
a) Ser/Thr Ligation (STL)
(Proc. Natl. Acad. Sci. , 2013)
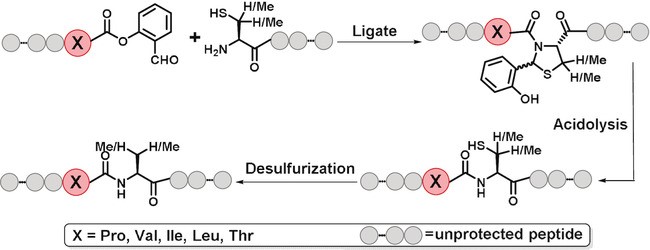
b) Cysteine/Penicillamine ligation (CPL)
(Angew. Chem. Int. Ed., 2020)
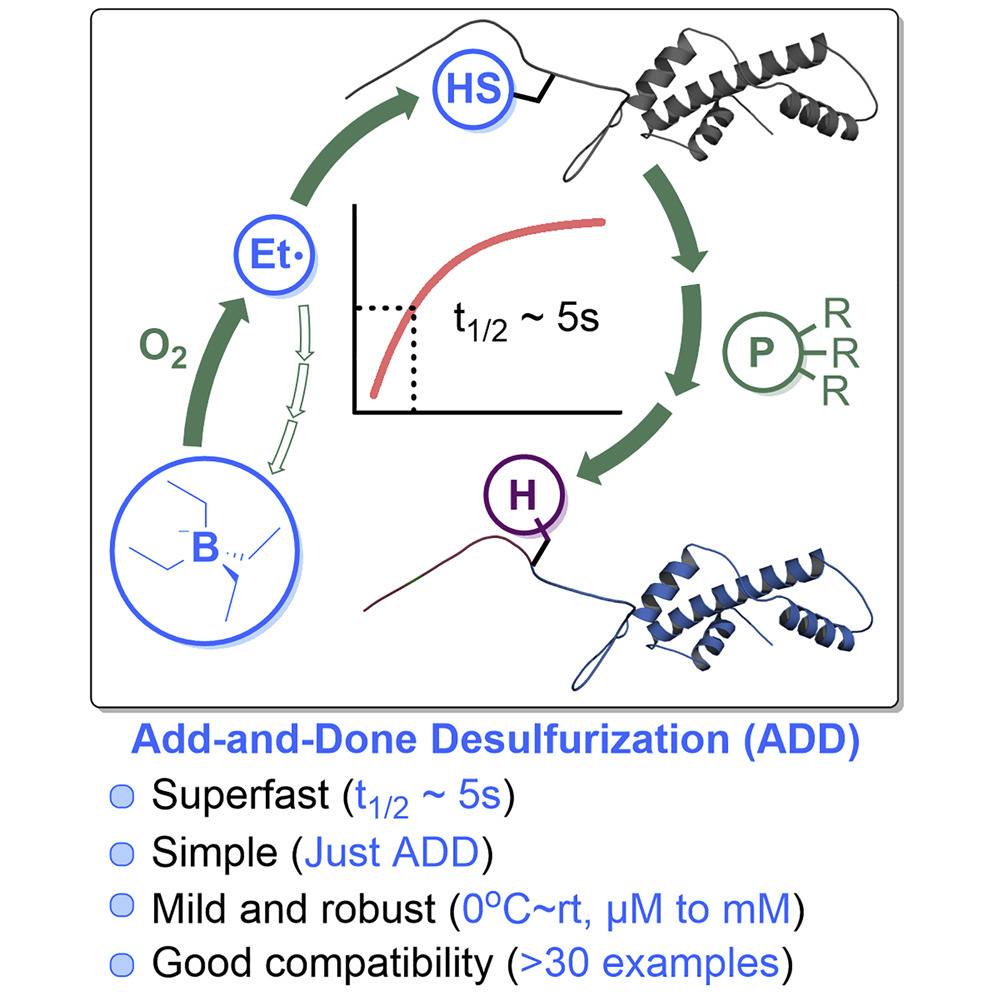
c) Superfast Desulfurization
(Chem 2022)
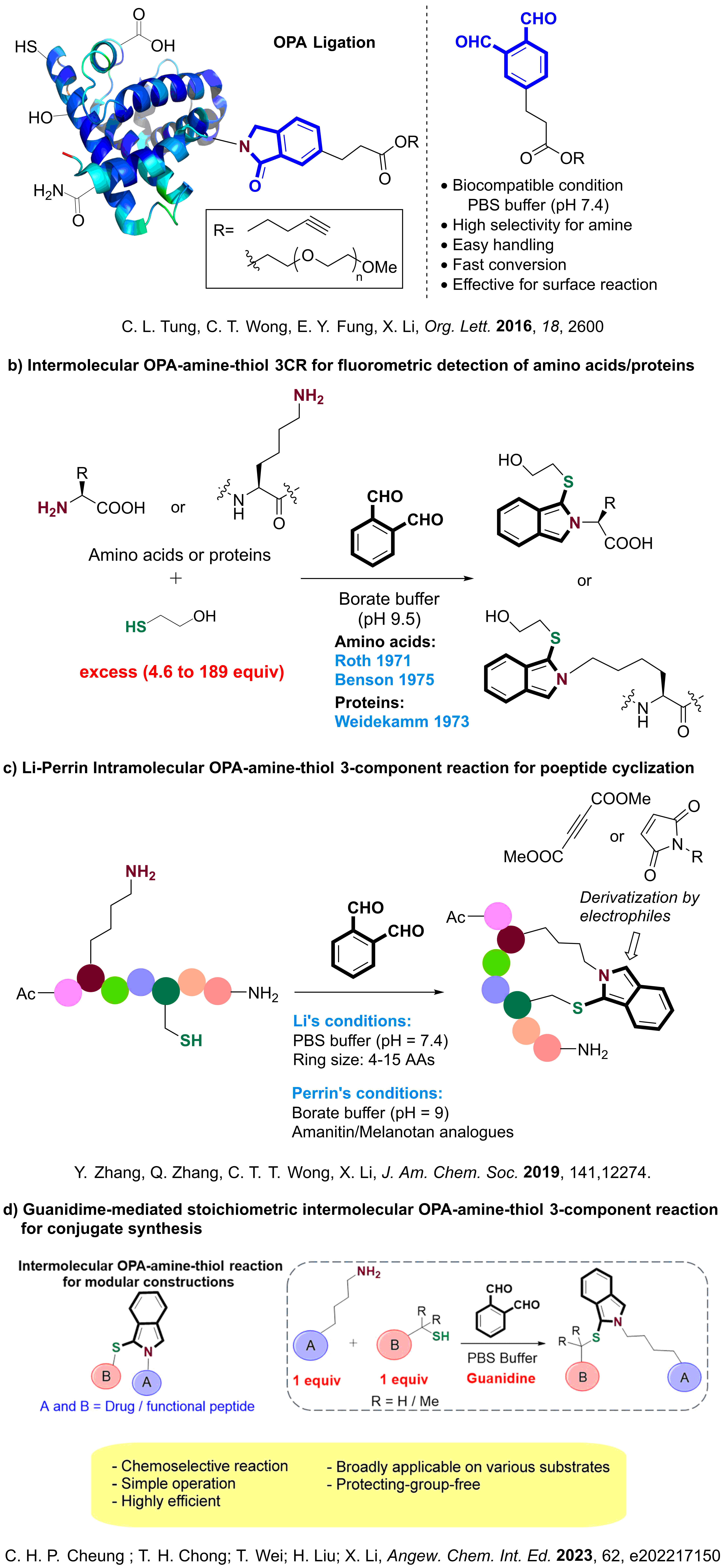
2) New OPA chemistry in peptide/protein science
3) Synthetic carbohydrate chemistry
a) Ring-Closing Glycosylation (RCG)
(J. Org. Chem. 2014)
II. Total synthesis and medicinal chemistry of natural products
1)Total synthesis of daptomycin
( J. Am. Chem. Soc., 2013)

J. Am. Chem. Soc. 2013, 135, 6272
J. Med. Chem. 2020, 63, 3161
ACS Med. Chem. Lett. 2020, 11, 1442
2) Total synthesis of teixobactin
(Nature Communications. , 2016)
Nature Commun. 2016, 7, 12394
Bioorg. Med. Chem. 2017, 17, 30578
Bioorg. Med. Chem. 2018, 26, 1062
3) Total synthesis of Antibiotics A54145
(Org. Lett. , 2019)
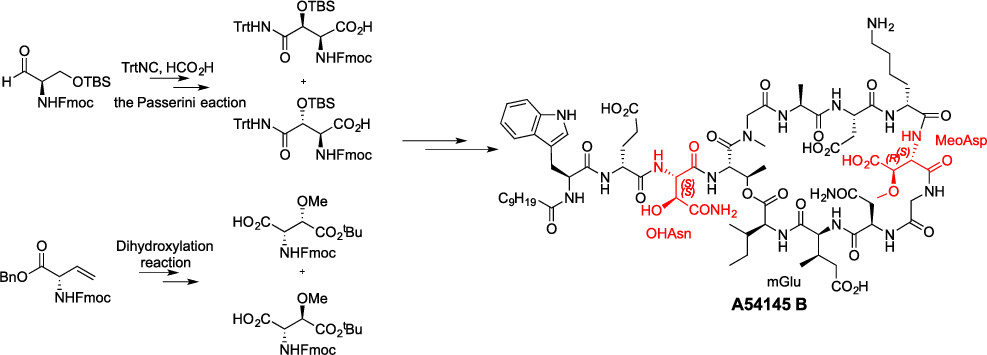
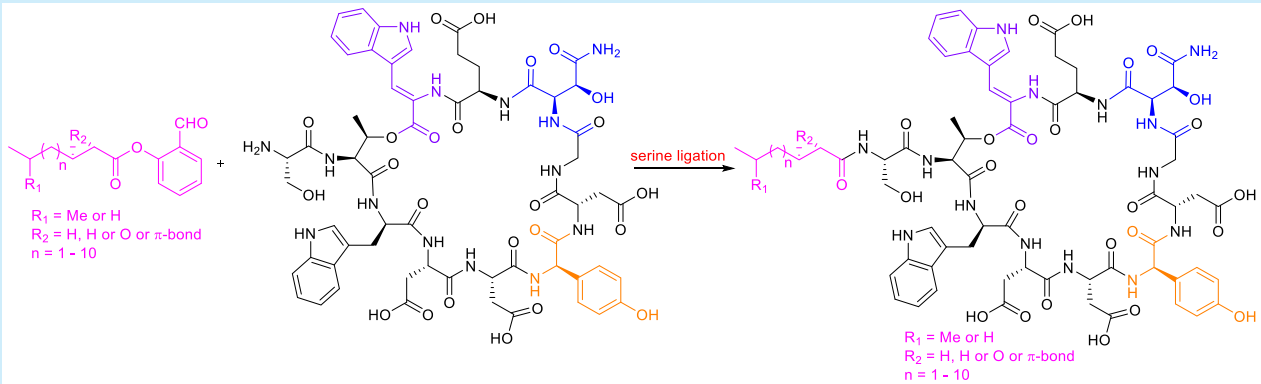
4) Total synthesis of Antibiotics CDA3a
(Org. Lett. , 2020)
5) Total synthesis of Malacidin A
(Angew. Chem. Int. Ed. , 2020)
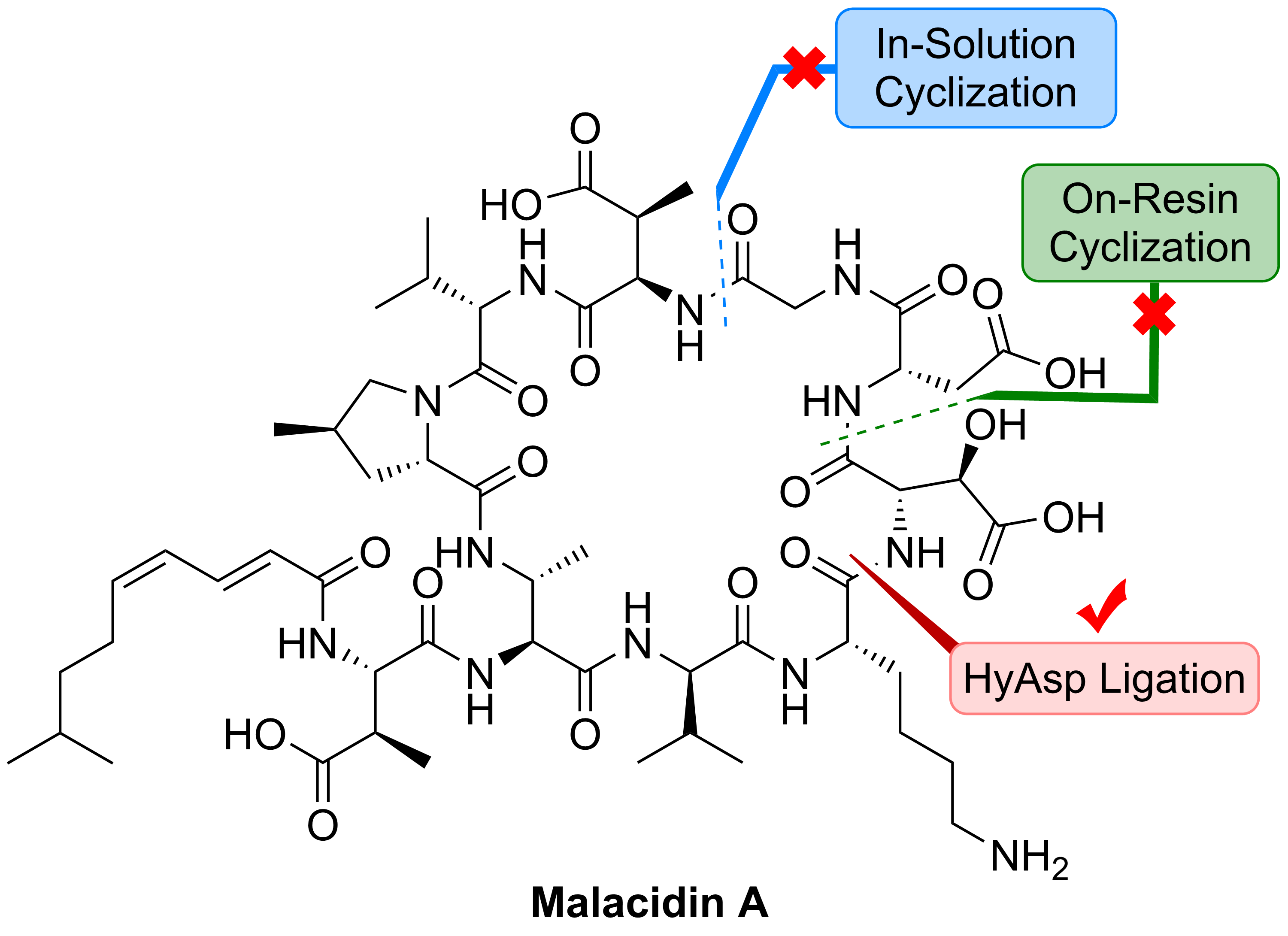
6)Total synthesis of mannopeptimycin
(J. Am. Chem. Soc., 2021)
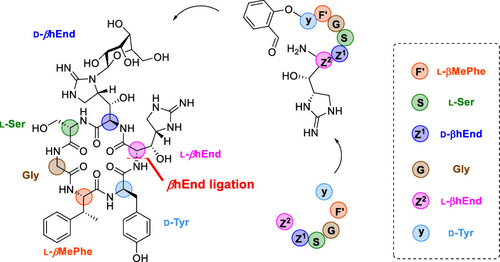
III. Chemical glycobiology of bacterial glycans
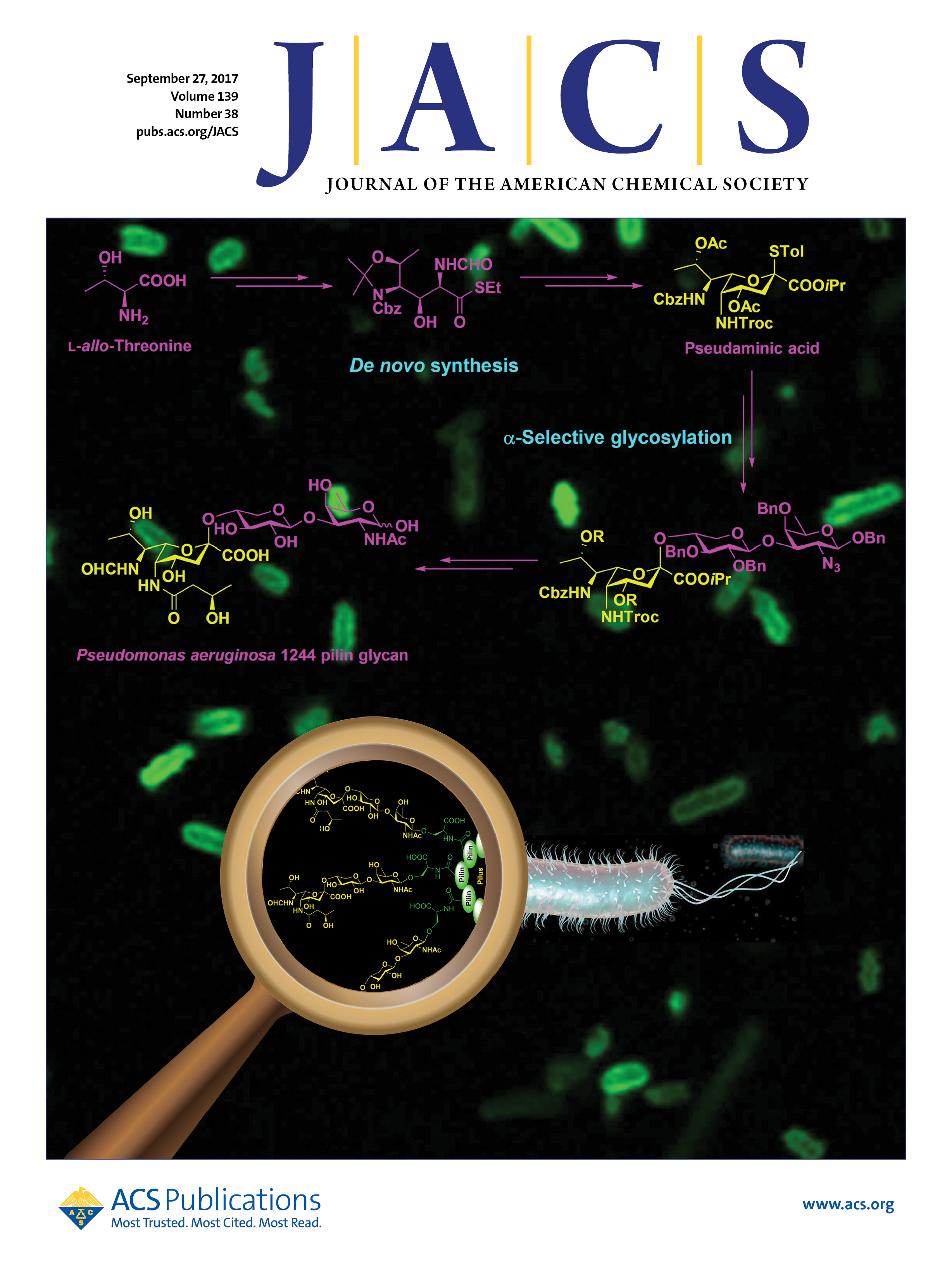
1)Total Synthesis of Pseudomonas aeruginosa 1244 Pilin Glycan
( J. Am. Chem. Soc., 2017)
2) A Solution to Chemical Pseudaminylation via a Bimodal Glycosyl Donor for Highly Stereocontrolled α- and β- Glycosylation
(Org. Lett. , 2019)

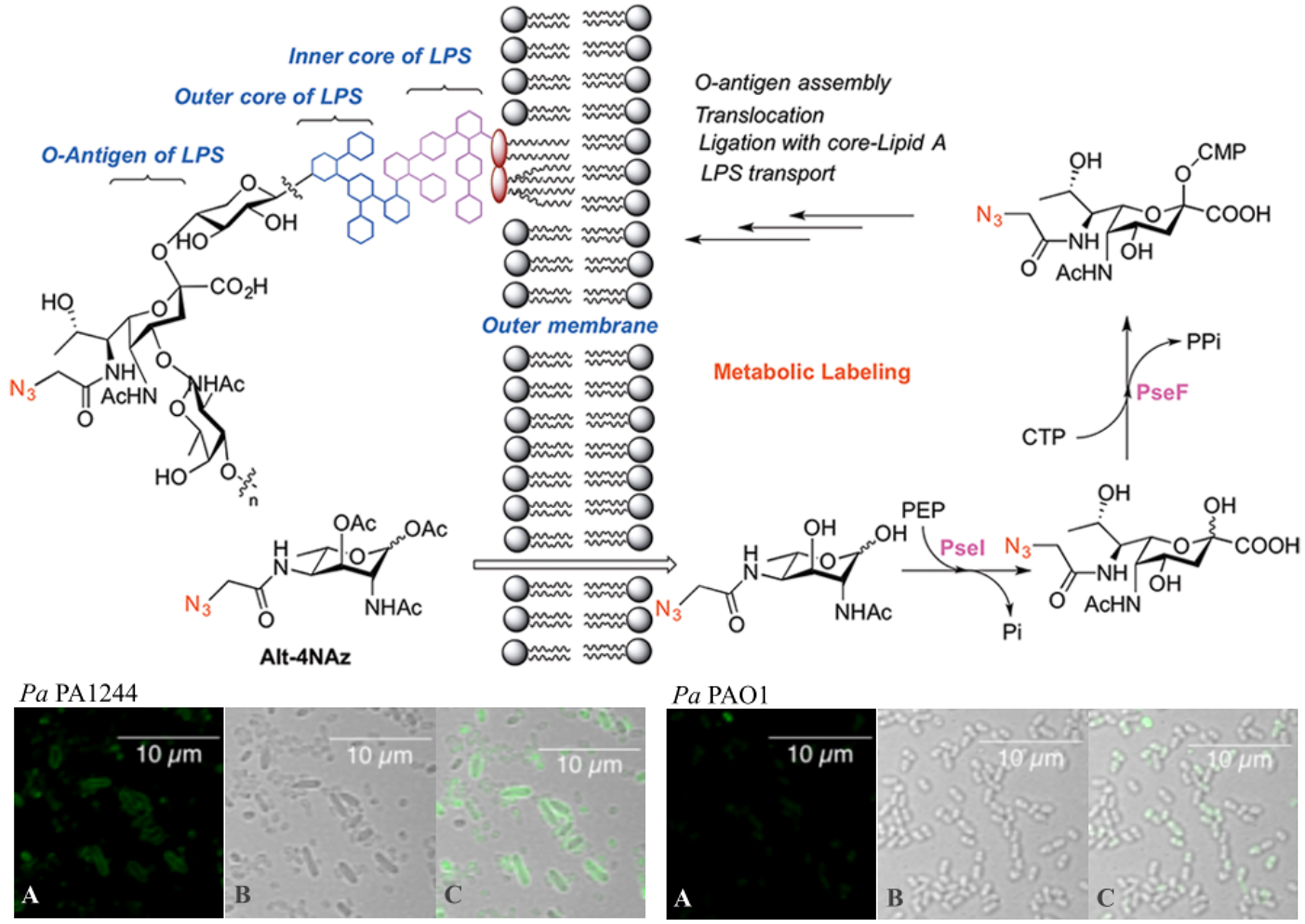
3) Bacterial Pse metabolic labeling
(ACS. Chem. Bio., 2018)
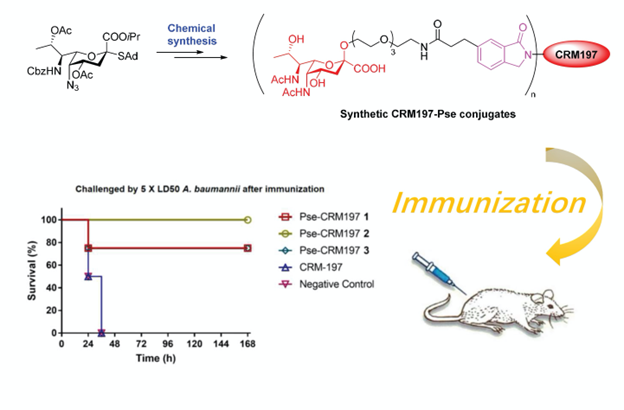
3) Synthetic Pse-based antibacterial vaccine
( Acs. Cent. Sci., 2021)
IV. Chemical biology of proteins with PTMs
1)HMGA1a
( Cell. Chem. Biol., 2021)


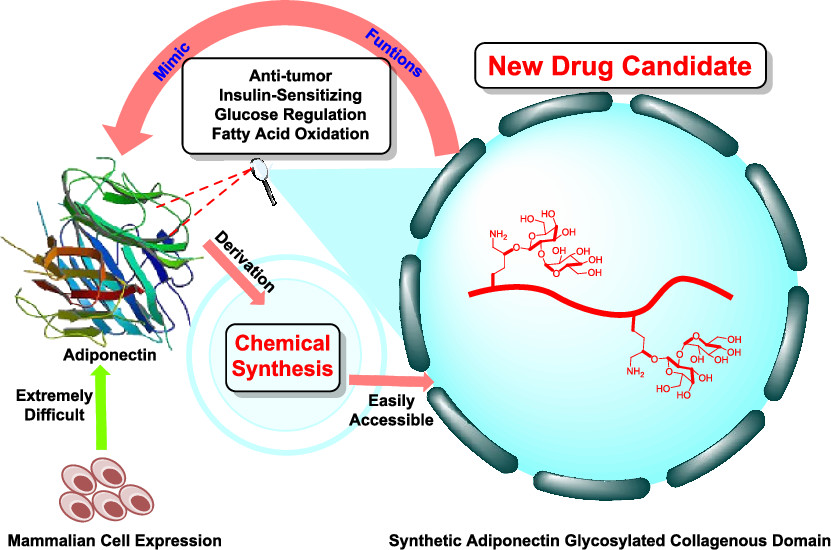
2) Adiponectin
(J. Am. Chem. Soc. , 2021)