
PROFESSOR K.Y. CHAN
 |
 |
 |
 |
 |
 |
Multiscale Structuring of Electrodes
Super-capacitors and fuel cells are energy storage and conversion devices critical to the success of renewable and clean energy technologies. Super-capacitors have high power density (500-10,000 W/kg) but low energy density (<10 Wh/kg) while fuel cells have higher energy density (>500 Wh/kg) but lower power density (<500W/kg). Both types of devices are electrochemical in nature and therefore limited by surface reactions requiring transport of charges towards and away from the interface. In addition to the specific chemistry of each system, surface area, pore sizes, and the network structure of the porous electrode dictate the rate of various charge transport processes. While much advance has been made in materials synthesis on the nanometer scale, a systematic study of how to integrate them into a larger scale porous electrode structure has been lacking. This project proposed to exploit recent template synthesis techniques to create carbon porous structures with macropores and mesopores organized together into a well-define structure, and to investigate their performance as electrocapacitance materials and fuel cell electrode materials. The investigation of how pores of multiple length scales integrate together for optimum charge and material transport is vital to the success of these energy technologies.
|
|
|
|
SEM image of mesoporous coated TentaGel
|
SEM Image of mesoporpus silica networked TentaGel
|
TEM image of CMK-8 mesoporous carbon
|
The electrocapacitance of CMK-8 mesoporous carbon in KOH |
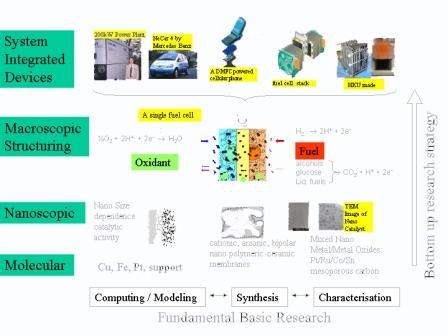
Fundamental and Applied Fuel Cell Research in Multiple Length Scales
[21] J. Ren, J. Ding, K.Y. Chan, and H.T. Wang, “Dual-Porosity Carbon Templated from Monosize Mesoporous Silica Nanoparticles”, Chem. Mater., 19 (2007) 2786.
[20] K.Y. Tsang, T.C. Lee, J.W. Ren, K.Y. Chan, H. Wang and H.T. Wang, “Platinum tungsten oxide (Pt-WO3) nanoparticles: their preparation in glycol and electrocatalytic properties”, J. Experimental Nanosci., 1 (2006) 113.
[19] K.Y. Tsang, T.C. Lee, J.W. Ren, K.Y. Chan, H. Wang and H.T. Wang, “Platinum tungsten oxide (Pt-WO3) nanoparticles: their preparation in glycol and electrocatalytic properties”, J. Experimental Nanosci., 1 (2006) 113.
[18] J. Ding, K.Y Chan, J.W. Ren, and F.S. Xiao, “Platinum and Platinum-Ruthenium Nanoparticles Supported on Ordered Mesoporous Carbon and their Electrocatalytic Performance for Fuel Cell Reactions”, Electrochim. Acta, 50 (2005) 3131.
[17] K.Y. Chan, J. Ding, J.W. Ren, S.A. Cheng and K.Y. Tsang, “Supported Mixed Metal Nanoparticles for Fuel Cell Electrode”, J. Mater. Chem., 14 (2004) 505.
[16] X. Zhang, K.Y. Tsang and K.Y. Chan, “Electrocatalytic Properties of Supported Platinum-Cobalt Nanoparticles with Uniform and Controlled Composition”, J. Electroanal. Chem., 573 (2004) 1.
[15] X. Zhang and K.Y. Chan, “Microemulsion Synthesis and Electrocatalytic Properties of Platinum- Ruthenium Nanoparticles”, Chem. Mater., 15 (2003) 451.
[14] X. Zhang and K.Y. Chan, “Microemulsion synthesis and electrocatalytic properties of platinum cobalt nanoparticles”, J. Mater. Chem., 12 (2002) 1203.
[13] N. Chi, K.-Y. Chan, and D.L. Phillips, "Electrocatalytic Oxidation of Formic Acid by Pt/Co Nanoparticles", Catalysis Letts, 71(2001) 21-26.
[12] I. Lee, K.-Y. Chan, and D.L. Phillips, "Atomic Force Microscopy of Platinum Nanoparticles Prepared on HOPG", Ultramicroscopy, 75 (1998) 69-76.
[11] I. Lee, K.Y. Chan, and D.L. Phillips, "Growth of Electrodeposited Platinum Nanocrystals Studied by Atomic Force Microscopy", Applied Surface Science, 136 (1998) 321.
[10] G. Wu and K.-Y. Chan, "Molecular Simulation of Oxygen on Supported Platinum Clusters", J. Electroanal. Chem., 450 (1998) 225.
[9] G. Wu and K.-Y. Chan, "Molecular Simulation of Platinum Clusters on Graphite", Surface Review and Letters, 4(5) (1997) 855-858.
[8] G. Wu and K.-Y. Chan, "Phase Behaviour of Oxygen adsorbed on Graphite", Fluid Phase Equilibria", 132 (1997) 21-31.
[7] G. Wu and K.-Y. Chan, "Morphology of Platinum Clusters on Graphite at Different Loadings", Surface Science, 365 (1996) 38-52.
[6] S. Liem and K.-Y. Chan, "Effective Pairwise Potential for Simulations of Absorbed Platinum", Mol. Phys., 86 (1995) 939-949.
[5] S. Liem and K.-Y. Chan, "Simulation Study of Platinum Adsorption on Graphite Using the Sutton-Chen Potential", Surface Science, 328 (1995) 119-128.
[4] S. Liem and K.-Y. Chan, "Morphology of Platinum Clusters between Graphite Walls", Mol. Simulation, 14 (1995) 125-136.
[3] S. Liem, K.-Y. Chan, and R.F. Savinell, "Molecular Dynamics Simulation of Platinum Particles between Graphite Walls", Mol. Simulation, 13 (1994) 47-60.
[2] X. Zhang, K.-Y. Chan, J.K. You, Z.G. Lin, A.C.C. Tseung, "Partial Oxidation of Glucose by Pt|WO3 Electrode", J. Electroanal. Chem., 430 (1997) 147-153.
[1] X. Zhang, K.-Y. Chan, and A.C.C. Tseung, "Electrochemical Oxidation of Glucose by Pt/WO3 Electrode", J. Electroanal. Chem., 386 (1995) 241-243.