PROFESSOR K.Y. CHAN
 |
 |
 |
 |
 |
 |
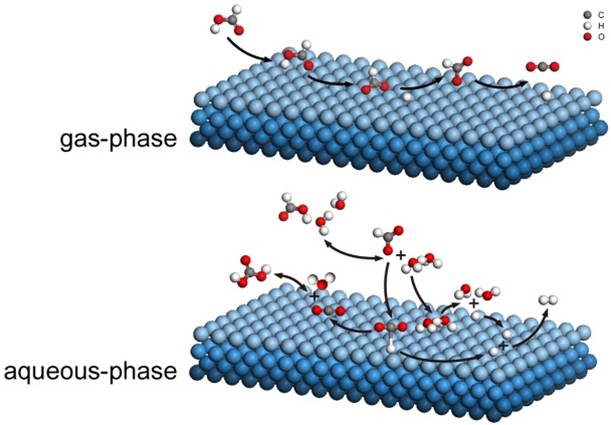
Reaction Pathway of formic acid decomposition on noble metals
Formic acid (HCOOH) has been proposed as a hydrogen storage media since the release of H2 without CO from HCOOH decomposition is attractive for PEM fuel cells. However, the reaction pathway of HCOOH decomposition on noble metals has not been well understood.
The present study proposed two reaction pathways from DFT computation for understanding HCOOH decomposition behavior on common noble metals. The key points for the reaction on the metals were clarified. This would enable us to rationally design more active heterogeneous catalyst for HCOOH decomposition to produce CO-free H2 at near ambient temperatures.